Part of Thermo Fisher Scientific
Material Safety Data Sheet
DIP SLIDE FOR CLINICAL USE
only available in certain territories
Code: DS0103, DS0102 & DS0120
Description
Kass (1956) defined significant urinary tract infection by a quantitative assessment of the bacterial population of freshly voided urine and was able to differentiate between infection and contamination. This led to many cultural and chemical methods being devised, to quantify the bacterial population in urine.
All these methods depend upon freshly voided urine being delivered promptly to the bacteriological laboratory for examination, either immediately or after a short period of refrigerated storage. Mackey and Sandys1,2 devised the method of filling a spoon with solid medium and using this as a dip-inoculum transport medium, thus avoiding misleading impressions produced by proliferation of organisms in urine between
collection and arrival at the laboratory.
The “Dip Slide” was developed as a logical extension of this method3. Using a microscope slide coated on each side with a different culture medium, larger areas of media could be used for bacterial assessment of urine. The glass slide was easy to contaminate, and there was also the risk of cuts if the glass was broken.
The Oxoid Dip Slide is made of easily disposable plastic. Its raised plastic edges ensure an even thickness of culture medium. The bottle cap is an integral part of the Dip Slide assembly, forming a convenient handle by which it may be held without risk of touching the culture medium It has a moulded grid which makes colony counting easier and is shaped to ensure adequate drainage of fluid from the sides.
Dip slides are simple and safe for an unskilled operator to use. The sterile Dip Slide can be inoculated on the ward or doctor’s surgery. It is then returned to its plastic container for transportation to the laboratory.
Technique
The aim is to wet both sides of the Oxoid Dip Slide with a mid-stream specimen of urine. This can be achieved in either of two ways:
- by collecting a mid-stream specimen in a sterile container and then dipping the Dip Slide in the urine.
- an alternative “dip-stream” procedure was developed by Arniel (1972).
The patient begins to urinate and after the stream is well established, the Dip Slide is held into it sufficiently to thoroughly wet each side.
The Dip Slide is held vertically for a moment to allow surplus urine to drain off the tip, and then returned to its sterile plastic container. Care must be taken not to touch the agar surface. The patient should be carefully instructed in the technique to follow.
The patient’s name and details of date and time etc. are noted on the label of the container and the Dip Slide is either posted or taken to a laboratory for subsequent incubation and examination. Samples sent through the post may already be positive on receipt in the laboratory. Apparently negative samples should be incubated at 37°C for at least 18 hours before being discarded.
| Code | Incubation | Culture Media |
| DS0102B & DS0103A | 35°C - 37°C for MacConkey Agar and CLED Medium | 18-48 hours |
| DS0120A | 35°C - 37°C for MacConkey Agar No. 3 and CLED Medium | 18-48 hours |
These media allow growth and identification of most of the bacteria likely to cause urinary tract infection. Reading and interpretation of the results should be carried out by an experienced microbiologist (see relevant pages on the Oxoid Web site for diagnostic characteristics).
Interpreation
Comparison of growth on Dip Slide 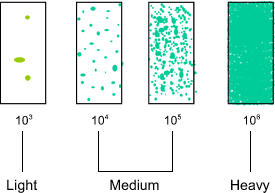
Kass (1956) stated that contaminant bacteria in fresh urine are fewer than 104 organisms per ml, whereas infecting organisms exceed 105/ml. Tests made with known bacterial suspensions of 104 per ml produce around 20 colonies on the Dip Slide. Suspensions containing 105 organisms per ml produce around 200 colonies. The Dip Slide, therefore, provides its maximum discrimination over the range of viable bacterial
counts which is most helpful in the diagnosis of urinary tract infections (Guttmann and Naylor). It is important to bear in mind, hwoever, that bacteria can be present in high number, without an infection being present, and diagnosis must be made on th basis of all the clinical evidence
Disposal of used Dip Slides
Used Dip Slides can be incinerated, autoclaved or soaked overnight in a suitable disinfectant, such as hypochlorite or a phenolic or quaternary ammonium compound product.
Storage of Dip Slides
Dip slides can be stored at 10–25ºC. Refrigeration at 4ºC is acceptable but may result in the release of water condensation. Do not use Dip Slides beyond the stated expiry date, or if the products show signs of deterioration.
1. Mackey and Sandys (1965) BMJ
2. Mackey and Sandys (1966) BMJ
3. Naylor, G.R.E., Guttmann, D. (1967) Cambridge Univ Press
Copyright, Disclaimer and Privacy Policy | Conditions of Sale | About Us | Cookies
Thermo Fisher Scientific Inc.

