Part of Thermo Fisher Scientific
Organisms
Organisms this product works with:
For this Organism
Other products used in the isolation of Escherichia coli:
Brilliance CRE AGAR
Code: PO1226 (Prepared media)
Brilliance™ CRE Agar is a chromogenic screening plate for the detection of carbapenem-resistant Enterobacteriaceae.
Only available in certain territories. Please speak to your local Oxoid supplier. Brilliance CRE Agar is not FDA cleared for sale in the US.
|
Typical formula* |
gm/litre |
|
Peptones |
15.0 |
|
Carbohydrates |
2.0 |
|
Titanium oxide |
5.1 |
|
Chromogenic mix |
1.0 |
|
Antibiotic mix |
19ml |
|
Agar |
15.0 |
* Adjusted as required to meet performance standards
Description
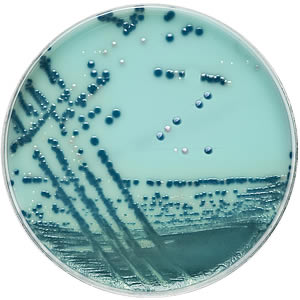 Brilliance™ CRE Agar provides presumptive chromogenic identification of carbapenem-resistant E. coli and the Klebsiella, Enterobacter, Serratia and Citrobacter (KESC) group, direct from clinical samples, in 18 hours.
Brilliance™ CRE Agar provides presumptive chromogenic identification of carbapenem-resistant E. coli and the Klebsiella, Enterobacter, Serratia and Citrobacter (KESC) group, direct from clinical samples, in 18 hours.
Carbapenems (imipenem, meropenem, ertapenem and doripenem) are invaluable for the treatment of multi-resistant Gram-negative bacterial infections, including producers of extended spectrum β- lactamases (ESBL)1. However, the rapid emergence and dissemination of Enterobacteriaceae resistant to carbapenems poses a considerable threat to clinical patient care and public health2. Early detection of carbapenem-resistant Enterobacteriaceae (CRE) will allow faster implementation of appropriate strategies to treat patients and limit the spread of these pathogens.
Traditionally, CRE detection has relied on antimicrobial susceptibility testing in conjunction with synergistic methods, like the Modified Hodge Test (MHT). However, MHT is technically demanding, and the results are often subjective and open to interpretation, so is no longer recommended by the CLSI or EUCAST. Also, these are confirmation tests and not suitable for patient screening, since primary culture must be conducted first.
As the number of CRE being isolated has increased, especially in geographies, such as India, Greece, Israel, Turkey and New York in the U.S.A., so had the need for an accurate, easy-to-use screening method to help identify colonized patient more quickly.
Brilliance CRE Agar makes detecting CRE-colonized patients easy. The formulation contains a modified carbapenem at a level recommended by both EUCAST and CLSI, ensuring reliable results with a wide variety of carbapenem-resistant organisms, including New Delhi Mettalo β-lactamase -1 (NDM-1).
The two-chromogen system differentiates E. coli (pale pink) from the KESC group organisms, which grow blue. Other carbapenem-resistant organisms not from the Enterobacteriaceae family, e.g. Acinetobacter, may grow, but they produce white or naturally pigmented colonies which are easy to spot against the novel turquoise background.
The novel background makes the white or naturally pigmented colonies of non-CRE organisms, like Acinetobacter, easy to spot, all of which means you can screen for CRE direct from patient samples in just 18 hours.
Plates are incubated at 37ºC and provide high sensitivity and specificity, with results available in just 18–24 hours. This allows a rapid response from infection control teams and enables the patient to receive the most appropriate treatment, as early as possible. Accuracy minimizes costs by ensure that only those in need receive what can be costly treatment.
Technique
Brilliance CRE Agar can be inoculated direct from rectal screening swabs, faecal samples or from an isolated colony prepared as a liquid suspension, approximately equivalent to 0.5 McFarland turbidity, according to local guidelines. The medium should be allowed to warm to room temperature before inoculation. Incubate for 18-24 hours at 37ºC. Negative plates should be re-incubated for an additional 24 hours. Pale pink colonies are presumptive positive for carbapenem-resistant E. coli and blue colonies for carbapenem-resistant KESC group. Resistant Proteus, Morganella and Providencia produce tan colonies with a brown halo through the tryptophan deaminase (TDA) reaction.
Escherichia coli identifications can be confirmed using RapID™ Spot Indole test (R8309002), or if subcultured on to a suitable medium, RapID ONE can be used to confirm speciation.
For further instructions on the use and interpretation of Brilliance CRE Agar, simply download the data sheet (2.04MB) in PDF format or visit www.oxoidhai.com/cre
Storage conditions and shelf life
Brilliance CRE Agar plates should be stored in the original packaging at the temperature stated on the pack or product specification, and protected from direct light. When stored as directed, the unopened product will remain stable until the expiry date on the label.
Appearance
Prepared medium: Pale, turquoise, semi-opaque gel medium in Petri dishes
Quality Control
Positive Controls |
Expected results |
|
Klebsiella pneumoniae NDM-1 NCTC13443 |
2mm, blue colonies |
|
Escherichia coli CRE (in-house strain) |
1–2mm, Pale pink colonies |
|
Acinetobacter baumannii NCTC13420 |
1–2mm, colorless to cream colonies |
|
Negative controls |
|
|
Enterococcus faecalis VRE ATCC® 51299 |
Complete inhibition |
|
Klebsiella pneumoniae ATCC® 29665 |
Complete inhibition |
|
Stenotrophomonas maltophilia ATCC® 136737 |
Complete inhibition |
|
Candida albicans ATCC® 10231 |
Complete inhibition |
Precautions
Brilliance CRE Agar is for in vitro diagnostic use only, by trained individuals. Do not use beyond the expiry date given on the label, or if the product shows any sign of deterioration. Sterilize specimens, equipment and media properly after use.
Limitations
Samples containing faecal material or blood may cause some localized discoloration within the medium. This discoloration should not be confused with a true chromogenic reaction where coloured colonies are visible.
References
- Department of Health Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection (ARHAI) and Health Protection Agency. 28 Jan 2011.
- Cohen S.J., Leverstein-Van Hall M.A., (2010) Int. J. Antimicrob. Agents 36, 205–210.
ATCC® is a registered trademark of American Type Culture Collection
Copyright, Disclaimer and Privacy Policy | Conditions of Sale | About Us | Cookies
Thermo Fisher Scientific Inc.

