Part of Thermo Fisher Scientific
Material Safety Data Sheet
Organisms
Organisms this product works with:
For this Organism
Other products used in the isolation of Staphylococcus sp:
SET-RPLA KIT TOXIN DETECTION KIT
Code: TD0900
Staphylococcal Enterotoxin Test Kit for the detection of staphylococcal enterotoxins A, B, C and D in food samples or culture filtrates by reversed passive latex agglutination
INTRODUCTION
Staphylococcal food poisoning is caused by eating foods contaminated with
enterotoxins produced during the growth of certain strains of Staphylococcus
aureus. Reports on the assay of these toxins by reversed passive latex agglutination
(RPLA) have been published. 1,2,3 The technique of reversed passive
latex agglutination (RPLA) enables soluble antigen such as bacterial toxins
to be detected in an agglutination assay.
In a standard agglutination assay, soluble antibody reacts with particulate
antigen such as bacterial cells. However, in a REVERSED agglutination
assay the antibody, which is attached to particles, reacts with the soluble
antigen. The particles (in this case, latex) do not themselves play a part in
the reaction and they are therefore PASSIVE. The cross-linking of the
latex particles by the specific antigen/antibody reaction results in the visible
LATEX AGGLUTINATION reaction.
The SET-RPLA test kit is based upon the reports by Shingaki et al.1
and Oda et al.4 It was developed under the guidance of the
Tokyo Metropolitan Research Laboratory of Public Health.
The SET-RPLA test may be used to detect staphylococcal enterotoxins in a wide
variety of foods and to give a semi-quantitative result. The test may also be
used to demonstrate enterotoxin production in isolates of S. aureus grown
in culture. It should be noted that coagulase-negative staphylococci have been
isolated which also produce enterotoxin in staphylococcal food poisoning.5
PRINCIPLE OF ASSAY
Polystyrene latex particles are sensitised with purified antiserum taken
from rabbits, immunised individually with purified staphylococcal enterotoxins
A, B, C and D. These latex particles will agglutinate in the presence of the
corresponding enterotoxin. A control reagent is provided which consists of latex
particles sensitised with non-immune rabbit globulins. The test is performed
in V-well microtitre plates. Dilutions of the food extract or culture filtrate
are made in five rows of wells, a volume of the appropriate latex suspension
is added to each well and the contents mixed. If staphylococcal enterotoxins
A, B, C or D are present, agglutination occurs, which results in the formation
of a lattice structure. Upon settling, this forms a diffuse layer on the base
of the well. If staphylococcal enterotoxins are absent or at a concentration
below the assay detection level, no such lattice structure can be formed and,
therefore a tight button will be observed.
The diluent provided contains sodium hexametaphosphate, which has been shown
to reduce the incidence of non-specific reactions with components of food matrices.6
PRECAUTIONS
This product is for in vitro diagnostic use only.
Do not freeze.
Reagents with different lot numbers should not be interchanged.
Reagents and diluent contain 0.1% sodium azide as a preservative. Sodium azide
may react with lead or copper plumbing to produce metal azides which are explosive
by contact detonation. To prevent azide accumulation in plumbing, flush with
copious amounts of water immediately after waste disposal.
STORAGE
The SET-RPLA Kit must be stored at 2-8° C. Under
these conditions the reagents will retain their reactivity until the date shown
on the kit box. After reconstitution, the enterotoxin controls should be stored
at 2-8° C. Under these conditions, the
reconstituted enterotoxin controls will retain the reactivity for 3 months,
or until the date shown on the kit box, whichever is the sooner.
SAMPLE PREPARATION
Food Matrices
A wide range of foods may be tested for staphylococcal enterotoxins; the
extraction procedure may, however, require modification for particular foods.
The main requirement is to achieve a non-turbid, fat-free extract. A low dilution
factor is desirable for optimum sensitivity, but if the nature of the food dictates
a greater dilution during extraction, a reduced sensitivity will result.
To gain a representative sample of a batch, a series of 10g portions are collected
from different locations within the batch (see T.P.I., U.S.D.A. sampling plans
or equivalent).
Culture Filtrates
Staphylococci from either clinical sources or food matrices may be recovered
and identified using suitable techniques described in standard textbooks.
METHOD OF USE
Materials required but not provided
Blender or homogeniser
Microtitre plates (V-well) and lids
Fixed or variable pipette and tips (25ml)
Centrifuge capable of generating 900g (typically 300 rpm in a small bench top
centrifuge)
Membrane filtration unit using low protein-binding disposable filters with a
porosity of 0.2mm-0.45mm
(such as Millipore SLGV)
Tryptone Soya Broth (CM129)
Sodium chloride solution (0.85%)
Sodium hypochlorite solution (>1.3% w/w)
25ml dropper (optional)
25ml diluter (optional)
Micromixer (optional)
Moisture box (optional)
Components of Kit
TD901 Latex sensitised with anti-enterotoxin A. Latex suspension sensitised
with specific antibodies (rabbit lgG) against staphylococcal enterotoxin A.
TD902 Latex sensitised with anti-enterotoxin B. Latex suspension sensitised
with specific antibodies (rabbit lgG) against staphylococcal enterotoxin B.
TD903 Latex sensitised with anti-enterotoxin C. Latex suspension sensitised
with specific antibodies (rabbit lgG) against staphylococcal enterotoxin C.
TD904 Latex sensitised with anti-enterotoxin D. Latex suspension sensitised
with specific antibodies (rabbit lgG) against staphylococcal enterotoxin D.
TD905 Latex control. Latex suspension sensitised with non-immune rabbit
globulins.
TD906 Staphylococcal enterotoxin A control
TD907 Staphylococcal enterotoxin B control.
TD908 Staphylococcal enterotoxin C control.
TD909 Staphylococcal enterotoxin D control.
TD910 Diluent. Phosphate buffered saline containing bovine serum albumin
and sodium hexametaphosphate.
Instruction leaflet
Toxin Extraction or Production
Extraction from Food Matrices
Blend 10g of sample with 10ml of sodium chloride solution (0.85%) in a blender
or homogeniser.
Centrifuge the blended sample at 900g at 4° C for
30 minutes. NOTE: If a refrigerated centrifuge is not available, cool
the sample to 4° C before centrifugation.
Filter the supernatant through a 0.2mm-0.45mm
low protein-binding membrane filter. Retain the filtrate for assay of toxin
content.
Production of Enterotoxins in Culture Fluids
Inoculate the isolated organism into Tryptone Soya Broth (CM129) and incubate
at 37° C for 18-24 hours, preferably with shaking.
After growth, either centrifuge at 900g for 20 minutes at 4°
C or membrane filter using a 0.2mm low protein-binding
filter. Retain the filtrate for assay of toxin content.
Control
Each reconstituted toxin control will cause agglutination with its respective
sensitised latex. The use of the toxin controls will provide references for
the positive patterns illustrated below (see interpretation of Test Results).
The controls should be used from time to time only to confirm the correct working
of the test latex. The toxin controls are not provided at a specified level
and therefore must not be used as a means of quantifying the level of toxin
detected in the test sample.
Assay Method
Working Reagents
The latex reagents and diluent are ready for use. The latex reagents should
be thoroughly shaken before use to ensure a homogeneous suspension. To reconstitute
the control reagents, add 0.5ml of diluent (TD910) to each vial. Shake gently
until the contents are dissolved.
Arrange the plate so that each row consists of 8 wells. Each sample needs the
use of 5 such rows.
Using a pipette or dropper, dispense 25ml of diluent
in each well of each of the 5 rows.
Using a pipette or dropper, dispense 25ml of test
sample to the first well of each of the 5 rows.
Using a pipette or diluter and starting at the first well of each row,
pick up 25ml and perform doubling dilutions along
each of the 5 rows. Stop at the 7th well to leave the last well containing
diluent only.
To each well in the first row, add 25ml of latex
sensitised with anti-enterotoxin A.
To each well in the second row, add 25ml of latex
sensitised with anti-enterotoxin B.
To each well in the third row, add 25ml of latex
sensitised with anti-enterotoxin C.
To each well in the fourth row, add 25ml of latex
sensitised with anti-enterotoxin D.
To each well in the fifth row, add 25ml of latex
control.
To mix the contents of each well, rotate the plate by micromixer or agitate
by hand. Take care that no spillage occurs from the wells.
To avoid evaporation, cover the plate with a lid. Placing the plate in a moisture
box is an acceptable alternative. Leave the plate undisturbed on a vibration-free
surface at room temperature for 20-24 hours. It will help subsequent reading
of the test if the plate is placed on black paper for the duration of this incubation.
Examine each well in each row for agglutination, against a black background.
Centrifuge tubes, membrane filters, microtitre plates, lids and pipette tips
should be sterilised by autoclaving at 121° C or
disinfected before disposal in hypochlorite solutions (>1.3% w/w).
Dispose of culture extracts, food extracts, samples and toxin controls in hypochlorite
solutions (>1.3% w/w).
INTERPREATION OF TEST RESULTS
The agglutination pattern should be judged by comparison with the following
illustration.
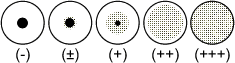
Results classified as (+), (++), and (+++) are considered to be positive.
Results in the row of wells containing latex control should be negative. In
some cases, non-specific agglutination may be observed. In such cases the results
should be interpreted as positive, provided that the reaction with sensitised
latex is positive to a higher dilution of test sample than that seen with the
latex control. The last well in all rows should be negative. If positive patterns
are observed in some of these wells, the reaction should be regarded as invalid.
NOTE: Certain staphylococcal strains are known to produce more than one
enterotoxin.
LIMITATIONS OF THE TEST
The sensitivity of this test in detecting the enterotoxins has been reported
to be 0.5ng/ml in the test extract. When a food extract is made with a dilution
ration of 1 : 1 with diluent, the sensitivity is, therefore, 1ng/g of food matrix.
The detection limit will vary according to any extra dilution conditions dictated
by the type of food matrix. Concentration of the enterotoxin in the food extract
can be effected by a variety of methods, such as ultrafiltration. Production
in culture of SETís depends on the growth conditions. A positive result obtained
by the culture demonstrates the production of one or more SET under those circumstances;
it does not imply the in vivo production of toxins to those levels.
REFERENCES
1. Shingaki,M et al. (1981). Ann. Rep. Tokyo Metro. Lab. Public
Health 32: 128.
2. Oda. T., et al. (1979) Ann. Rep. Fukuoka City Lab Hyg. 4
: 33.
3. Park, C and Szabo, R. (1986). Can. J. Microbiol. 32 : 723.
4. Oda, T. (1978) Jap. J. Bacteriol. 33: 743.
5. Crass, B and Bergdoll, M (1986). J Clin. Microbiol. 23: 43.
6. Rose, S., Bankes, P. and Stringer, M. (1989) Int. J Food Microbiol.
8: 65-72
Copyright, Disclaimer and Privacy Policy | Conditions of Sale | About Us | Cookies
Thermo Fisher Scientific Inc.

