Part of Thermo Fisher Scientific
Organisms
Organisms this product works with:
For this Organism
Other products used in the isolation of Yeasts:
BAX® SYSTEM Q7 FROM DUPONT QUALICON
The BAX® System from DuPont Qualicon allows food laboratories to quickly and accurately detect bacteria in raw ingredients, finished products and environmental samples.
Description
BAX® System Q7 from DuPont Qualicon is distributed exclusively alongside the Oxoid range throughout Europe, Australia, New Zealand and Canada. The award-winning BAX® system is a fast and accurate method for detecting pathogens and other organisms in food and environmental samples. The system breaks down samples at the genetic level, using the power of the polymerase chain reaction (PCR) to detect bacteria and other microbes with certainty.
The BAX® System allows food laboratories to quickly and accurately detect bacteria in raw ingredients, finished products and environmental samples. The cycler/detector allows you to load your prepared samples and walk away – interpreted positive/negative results are clearly displayed on-screen, raw data easily accessible.
| Product Description |
Order Code |
|
Automated BAX® system Q7 start up package; PCR Cycler/ Detector, Computer/Monitor/ Keyboard/ Mouse/Printer; BAX software/Microsoft® Windows® based, Dry Block Heaters (2), Cooling Blocks (3), Capping/Decapping Tools, Pipettes, Disposables to run the system, 1 year warranty. |
QB0003H |
| BAX® System Salmonella Assay | QB0608C |
| BAX® System Listeria monocytogenes 24E Assay | QB8125C |
| BAX® System Genus Listeria 24E Assay | QB8135C |
| BAX® System Real-Time Escherichia coli O157:H7 Assay | QB3648C |
| BAX® System Escherichia coli O157:H7 MP Assay | QB0673C |
| BAX® System Real-Time Campylobacter coli/jejuni/lari Assay | QB3449C |
| BAX® System Enterobacter sakazakii Assay | QB0657C |
| BAX® System Real-Time Staphylococcus aureus Assay | QB2689C |
| BAX® System Vibrio Real-Time Vibrio cholerae/parahaemolyticus/vulnificus | QB3877C |
|
BAX® System Real-Time STEC Assay Suite: (NEW) |
|
| QB2964C | |
| QB2970P | |
| BAX® System Real-Time STEC Panel 2 Assay (O45, O103, O145) | QB2987P |
| BAX® System Yeast and Mould Assay | QB0682C |
Advanced technology with the BAX® System Q7
Incorporating innovative PCR technology, the new BAX® System Q7 offers advanced DNA-based detection for a broad range of sampling applications - from ingredients to finished products.
With a single program for most targets, the BAX® system allows you to test multiple targets in the same run, up to 96 samples per batch. Tableted reagents, which enable minimal hands-on time using standard laboratory techniques, provide long shelf life and consistency. User friendly screen prompts guide you through the entire procedure, reducing the need for highly skilled technicians and expensive training. Including sample enrichment, most assay results are available next day. Results are clearly displayed on-screen with a simple positive or negative icon that virtually eliminates the need for expert interpretation. Electronic files allow you to print, share and store your results for easy archiving and retrieval.
Customers around the world have made the BAX® system an integral part of their quality assurance systems due to its tremendous impact on their operations - from dramatically decreasing false positives and minimizing re-testing, to reducing employee training and speeding product time to market. The speed and accuracy of DNA-based detection, including a license to perform PCR; the simplicity of a load-it and leave-it operation; the familiarity of a Microsoft® Windows® interface: The BAX® system combines it all for smooth testing procedures at any stage in the processing pipeline.
How the BAX® System Works
The BAX® System process consists of preparation of enriched samples, followed by automated amplification and detection. These steps use simplified molecular biology techniques and require no additional specialized skills.
 Samples are enriched according to standard protocols for the food type. Samples are then heated in a lysis reagent solution to rupture the bacterial cell wall and release the DNA.
Samples are enriched according to standard protocols for the food type. Samples are then heated in a lysis reagent solution to rupture the bacterial cell wall and release the DNA.
 PCR tablets, which contain all the reagents necessary for PCR plus fluorescent dye, are hydrated with lysed sample and processed in the cycler/detector. Within a few hours, the polymerase chain reaction (PCR) amplifies a DNA fragment that is specific to the target.
PCR tablets, which contain all the reagents necessary for PCR plus fluorescent dye, are hydrated with lysed sample and processed in the cycler/detector. Within a few hours, the polymerase chain reaction (PCR) amplifies a DNA fragment that is specific to the target.
 The amplified DNA generates a fluorescent signal, which the BAX® System application uses to analyse the findings. Results are then displayed on your monitor screen as simple positive or negative symbols.
The amplified DNA generates a fluorescent signal, which the BAX® System application uses to analyse the findings. Results are then displayed on your monitor screen as simple positive or negative symbols.
What is PCR?
The Polymerase Chain Reaction (PCR) involves the amplification of target-specific DNA to detectable levels. The process requires, primers (short sequences of DNA that are complementary to the target DNA), DNA polymerase (an enzyme to catalyse the reaction) and free nucleotides that are used in the extension of the amplified DNA. This process is strictly controlled by raising and lowering the temperature and is repeated 30 – 40 times to allow amplification. This process is known as thermo-cycling.
Target specific DNA is extracted from target cells by heating the sample with a lysing agent. Cell walls are broken down and the double strands of DNA are then denatured (unwound and separated by heating to 90-96ºC) into single stranded DNA. After cooling to about 55ºC, primers then anneal to the separated target DNA. The temperature is then raised to 72ºC and DNA synthesis occurs as polymerase extends the new sequence along the single strand of target DNA, using the free nucleotides as DNA building blocks. This results in two double strands of DNA – identical copies of the original target DNA. Multiple repeats of this denaturation, annealing and extension process (thermo-cycling) results in an exponential increase in the concentration of target DNA (figure 1). This results in sufficient genetic material for accurate and reliable detection in a matter of hours.
Figure 1. The Polymerase Chain Reaction (PCR).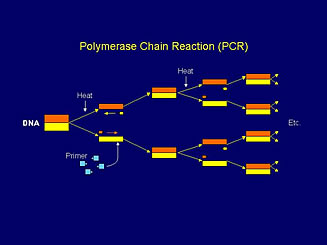
In the past, polymerase had to be added for each cycle as the high temperatures required for separating the DNA strands also destroyed the enzyme. However, the discovery of a thermo-stable enzyme, Taq polymerase, allowed PCR to be automated and the potential for the technique in many different applications exploded.
Automated cycler-detectors have permitted the development of walk-away pathogen detection systems, such as the BAX® System Q7 from DuPont Qualicon. Developments in PCR detection techniques have also ensured that the amplified DNA is contained within the test unit for the duration of the process. This reduces the risk of cross contamination and eliminates the requirement for a separate work area.
There are two types of PCR detection systems currently available:
- Real-time PCR offers a quantifiable result, relating back to the amount of DNA in the specimen as it was loaded into the cycler-detector. Real-time PCR detects the amplified DNA during the highly precise exponential phase of PCR using special fluorescent probes. It collects data at the end of each cycle, providing a faster, method for DNA quantification. Use of different fluorescent probes enables real-time PCR to differentiate between multiple targets.
- End-point PCR offers a qualitative result. Detection takes place when the PCR process is complete. Intercalating dyes, which fluoresce when incorporated into a double stranded section of (specific) DNA, are used in the detection process. A simple yes/no result is given at the end.
The BAX® System Q7 is an automated PCR system which allows both end-point and real-time assays to be run. It offers a real-time assay for the detection, differentiation and quantification of Campylobacter species (C. jejuni, C. coli and C. lari) and Vibrio species (V. cholorae, V. parahaemolyticus and V. vulnificus) in the same sample; detection and threshold quantification of Staphylococus aureus and clear end-point results for other important food-borne pathogens.
The BAX® System is used successfully in many different food microbiology laboratories. It has been validated or certified by AFNOR, AOAC and other international bodies to test a wide variety of food products (depending on the target organism), including dairy, meat, seafood, chocolate, fruit and fruit juices, vegetables, salads, and animal feeds.1
- Full details of validations, approvals and adoptions are available from your Oxoid product supplier or from the DuPont Qualicon website.
Copyright, Disclaimer and Privacy Policy | Conditions of Sale | About Us | Cookies
Thermo Fisher Scientific Inc.

